Journal of Energy Chemistry, 2024
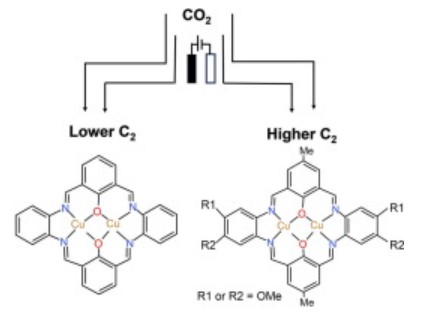
Molecular copper catalysts serve as exemplary models for correlating the structure-reaction-mechanism relationship in the electrochemical CO2 reduction reaction (eCO2R), owing to their adaptable environments surrounding the copper metal centres. This investigation, employing density functional theory (DFT) calculations, focuses on a novel family of binuclear Cu molecular catalysts. The modulation of their coordination configuration through the introduction of organic groups aims to assess their efficacy in converting CO2 to C2 products. Our findings highlight the crucial role of chemical valence state in shaping the characteristics of binuclear Cu catalysts, consequently influencing the eCO2R behaviour. Notably, the Cu(II)Cu(II) macrocycle catalyst exhibits enhanced suppression of the hydrogen evolution reaction (HER), facilitating proton transfer and the eCO2R process. Furthermore, we explore the impact of diverse electron-withdrawing and electron-donating groups coordinated to the macrocycle (R = –F, –H, and –OCH3) on the electron distribution in the molecular catalysts. Strategic placement of –OCH3 groups in the macrocycles leads to a favourable oxidation state of the Cu centres and subsequent C–C coupling to form C2 products. This research provides fundamental insights into the design and optimization of binuclear Cu molecular catalysts for the electrochemical conversion of CO2 to value-added C2 products.