Phys. Chem. Chem. Phys., 2017,19, 2409-2416
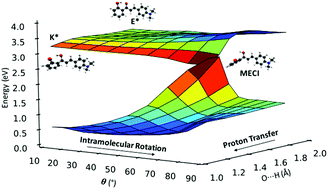
Fluorophores exhibiting excited-state intramolecular proton transfer (ESIPT) are promising candidates for applications ranging from imaging and probing to laser dyes, optoelectronic devices and molecular logic gates. Recently, ESIPT-active solid-state emitters based on 2′-hydroxychalcone have been synthesized. The compounds are almost non-emissive in solution but emit in the deep red/NIR region when crystalline. Herein, we present a comprehensive theoretical investigation of the gas-phase excited state relaxation pathways in five 2′-hydroxychalcone systems, using a combination of static and non-adiabatic simulations. We identify two competing non-radiative relaxation channels, driven by intramolecular rotation in the enol and keto excited states. Both mechanisms are accessible for the five compounds studied and their relative population depends on the nature of the substituent. The addition of electron-donating substituents greatly increases the propensity of the ESIPT pathway versus rotation in the enol state. The identification of the fundamental relaxation mechanisms is the first step towards understanding the aggregated emission phenomena of these compounds.